Recent meeting abstracts
IL-15-stimulated NK cell proliferation is greatly augmented by the anti-ADAM17 mAb Medi-1 and requires CD137
Publication highlights
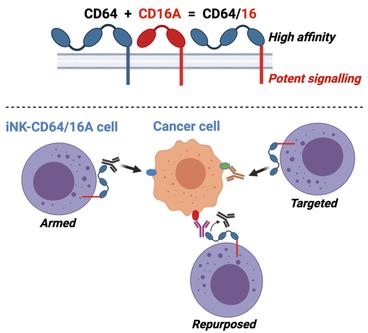
iPSC-derived natural killer cells expressing the FcγR fusion CD64/16A can be armed with antibodies for multitumor antigen targeting.
Snyder KM, Dixon KJ, Davis Z, Hosking M, Hart G, Khaw M, Matson A, Bjordahl R, Hancock B, Shirinbak S, Miller JS, Valamehr B, Wu J, Walcheck B., In: Dec 6 2023, J Immunother Cancer
An ADAM17-Neutralizing Antibody Reduces Inflammation and Mortality While Increasing Viral Burden in a COVID-19 Mouse Model
Jodi F. Hedges, Deann T. Snyder, Amanda Robison, Heather M. Grifka-Walk, Karlin Blackwell, Kelly Shepardson, Douglas Kominsky, Agnieszka Rynda-Apple, Bruce Walcheck, Mark A. Jutila, In: Jun 10 2022, Frontiers in immunology.
Examination of IgG Fc Receptor CD16A and CD64 Expression by Canine Leukocytes and Their ADCC Activity in Engineered NK Cells
Robert Hullsiek, Yunfang Li, Kristin M. Snyder, Sam Wang, Da Di, Antonella Borgatti, Chae Lee, Peter F. Moore, Cong Zhu, Chiara Fattori, Jaime F. Modiano, Jianming Wu, Bruce Walcheck, In: Feb 24, 2022, Frontiers in immunology.
Activation of ADAM17 by IL-15 Limits Human NK Cell Proliferation
Mishra, H. K., Dixon, K., Pore, N., Felices, M., Miller, J. S. & Walcheck, B., Jul 22 2021, In: Frontiers in immunology. 12, 711621.
Research output: Contribution to journal › Article › peer-review
Engineering anti-tumor monoclonal antibodies and fc receptors to enhance ADCC by human NK cells
Dixon, K. J., Wu, J. & Walcheck, B., Jan 16 2021, In: Cancers. 13, 2, p. 1-13 13 p., 312.
Research output: Contribution to journal › Review article › peer-review
Ectodomain shedding by ADAM17 (a disintegrin and metalloproteinase 17) in canine neutrophils
Snyder, K. M. , McAloney, C. A. , Montel, J. S. , Modiano, J. F. & Walcheck, B. , Jan 1 2021 , In: Veterinary immunology and immunopathology. 231 , 110162. Research output : Contribution to journal › Article › peer-review
Blocking adam17 function with a monoclonal antibody improves sepsis survival in a murine model of polymicrobial sepsis
Hemant K. Mishra, Jing Ma, Daniel Mendez, Robert Hullsiek, Nabendu Pore, Bruce Walcheck, In: Int. J. Mol. Sci. 2020, 21(18), 6688; https://doi.org/10.3390/ijms2118668